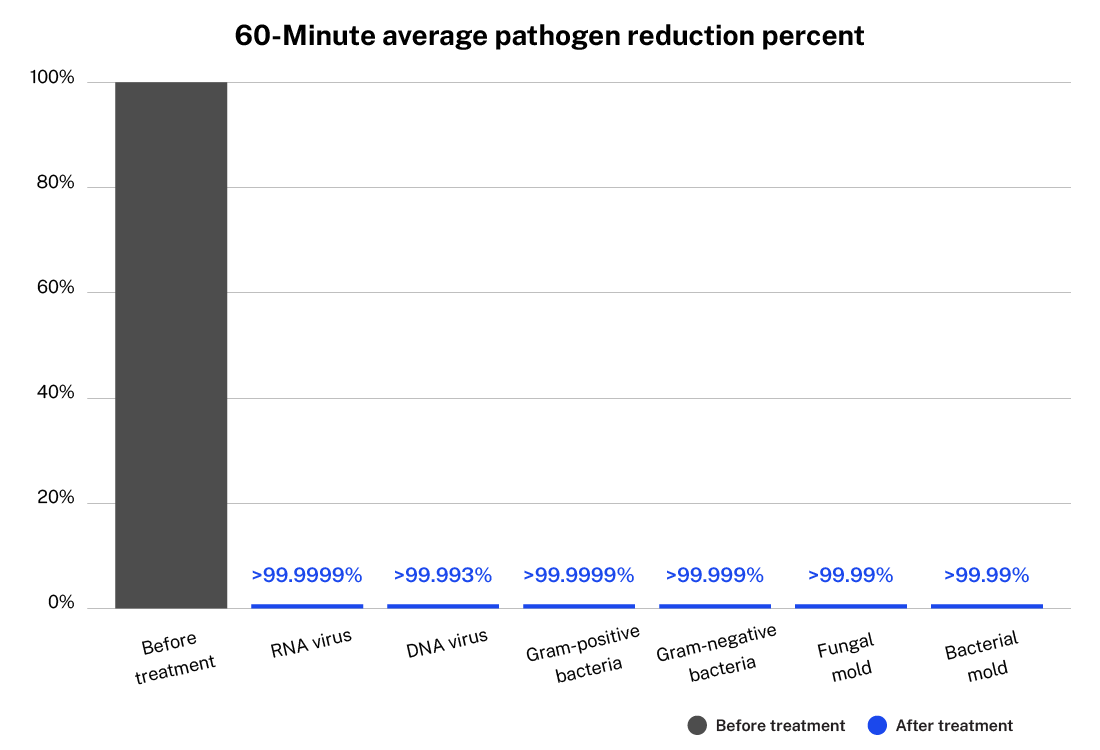
The An extensive unaffiliated labs including Dubai Central Laboratory and USA military labs have been tested ActivePure Technology to significantly reduces numerous contaminants and pathogens including the following (approximately 99.7%):
| Pathogen | Type |
|---|---|
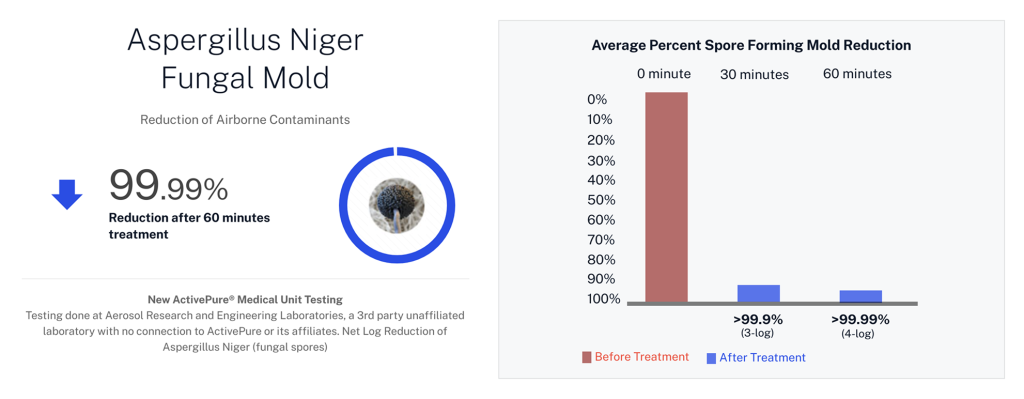
| Aspergillus niger endospores | Toxic Black Mold surrogate |
| Aspergillus versicolor | Fungus |
| Bacillus globigii | Bacterial endospore; Anthrax surrogate |
| Botrytis cinerea | Fungus |
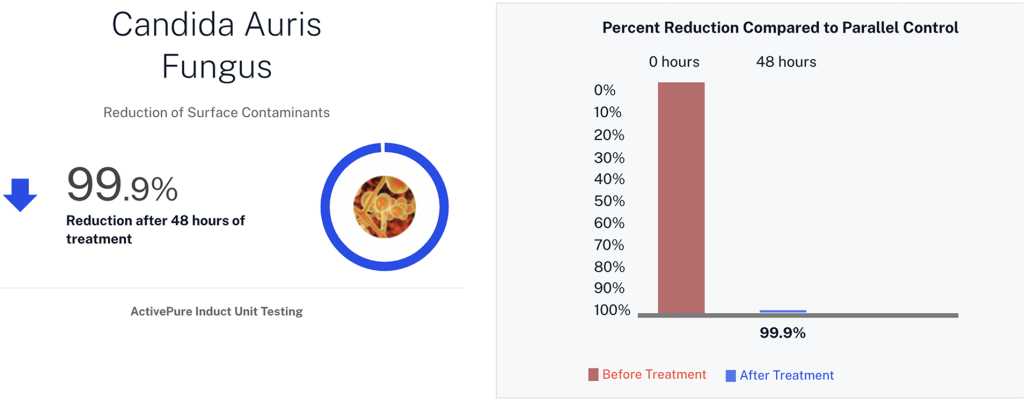
| Candida auris | Fungus |
| Clostridioides difficile | C. Diff; Endospore |
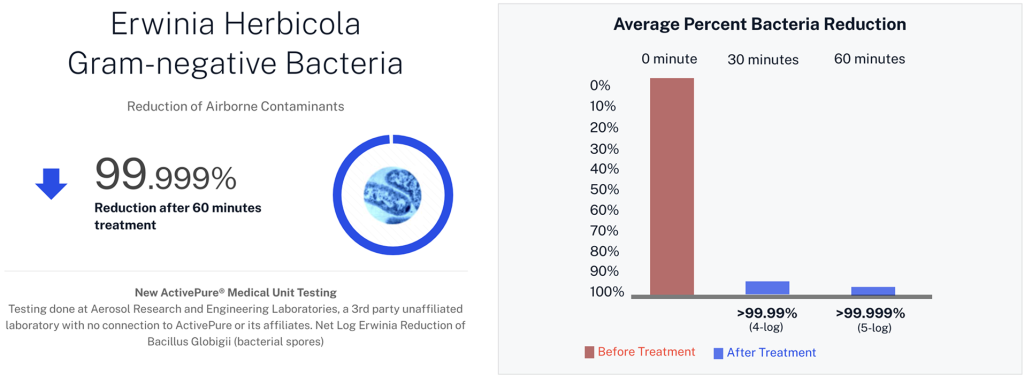
| Erwinia herbicola | Gram-bacteria |
| Escherichia coli | E. coli; Gram-bacteria |
| H1N1 | Swine Flu; Influenza A virus |
| H5N8 | Bird Flu; Influenza A virus |
| Legionella pneumophila | Gram-bacteria |
| Listeria monocytogenes | Gram+ bacteria |
| Methicillin-Resistant Staphylococcus aureus | MRSA; Gram+ bacteria |
| Monkeypox virus (MPXV) | Virus |
| Murine Norovirus | Virus |
| Mycobacterium tuberculosis | TB |
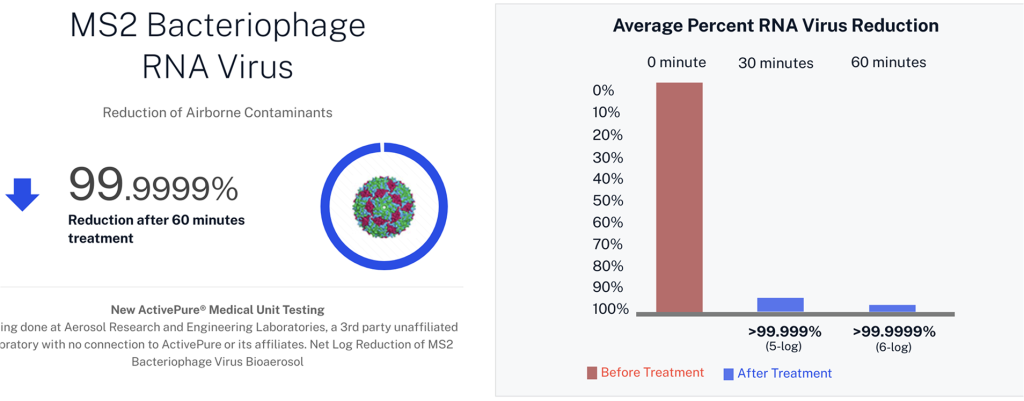
| MS2 | RNA bacteriophage virus |
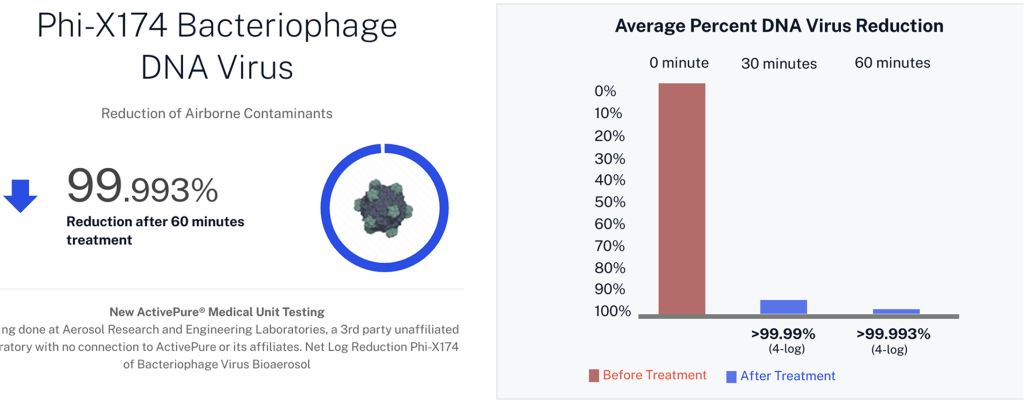
| Phi-X174 | DNA bacteriophage virus |
| Respiratory syncytial virus (RSV) | Virus |
| Salmonella enterica | Gram-bacteria |
| SARS-CoV-2 | Virus |
| Sclerotinia sclerotiorum | Fungus |
| Staphylococcus aureus | Gram+ bacteria |
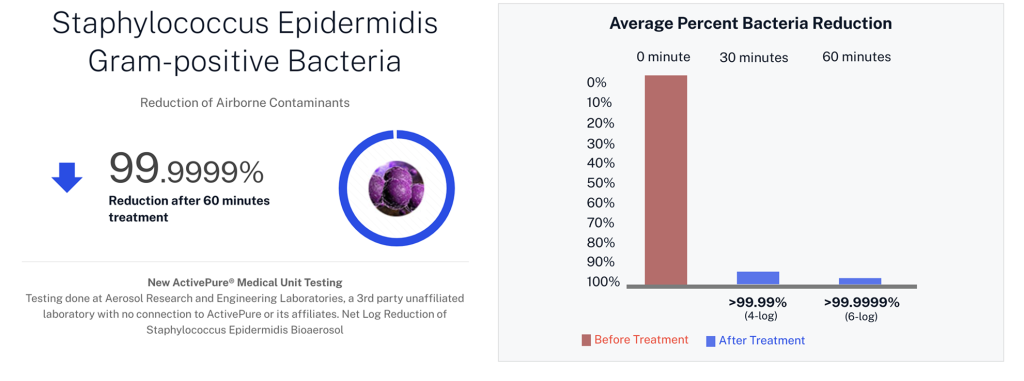
| Staphylococcus epidermidis | Gram+ bacteria |

Read the Report >>
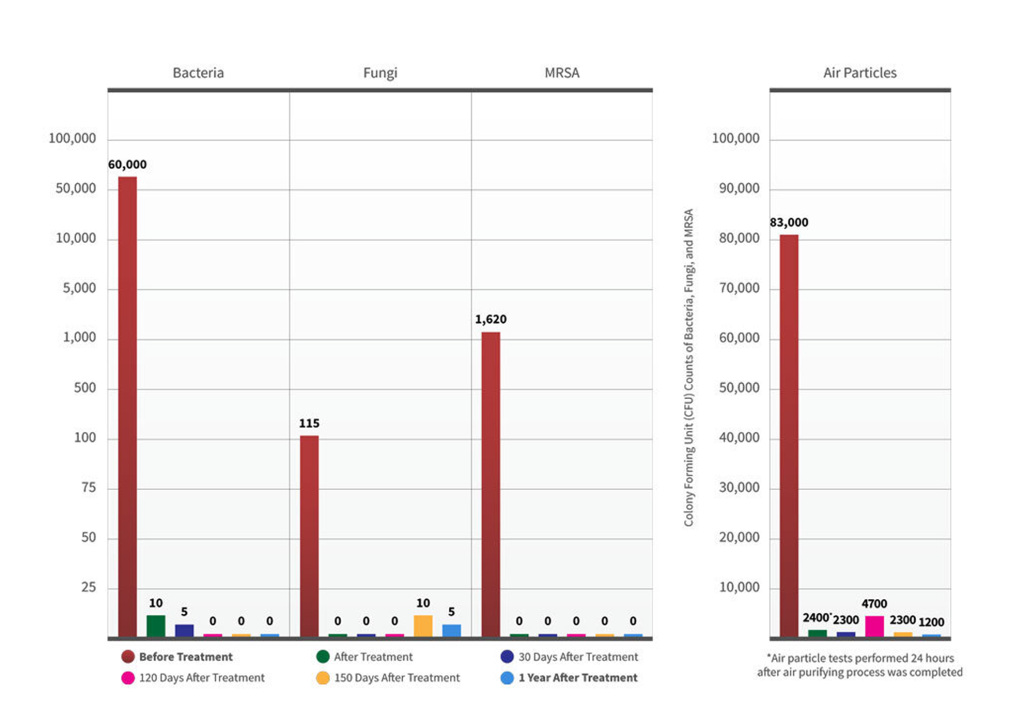
Total bacterial count
Reduction percentage
(Air Quality Test)
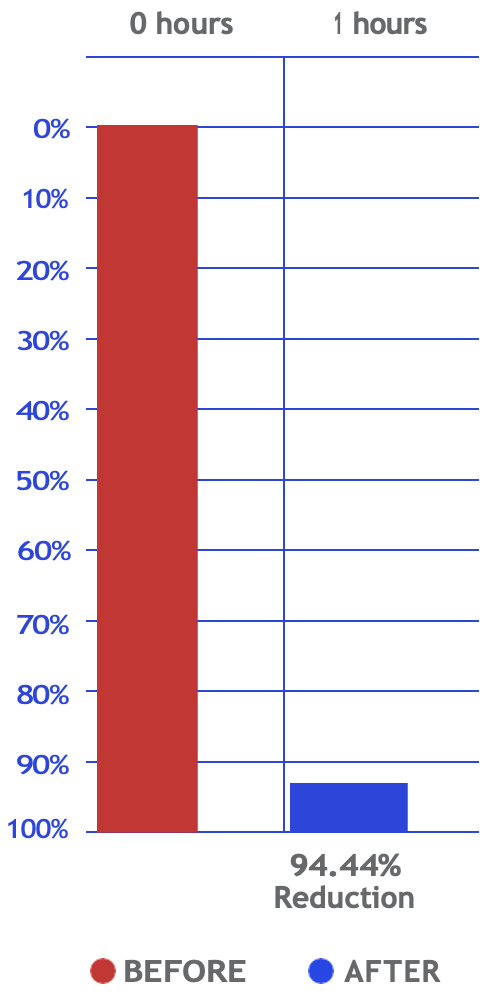
Yeast
Reduction percentage
(Air Quality Test)
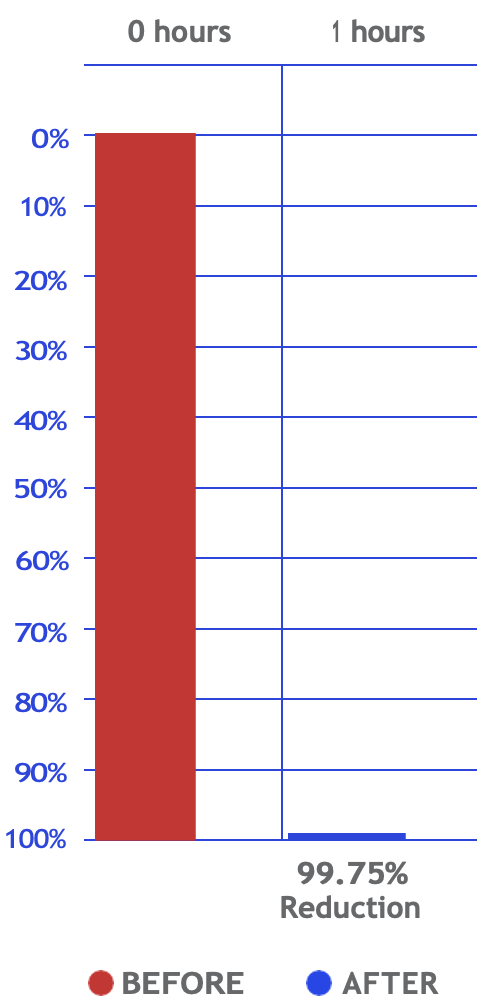
Bacteria
Reduction percentage
(Surface Test)
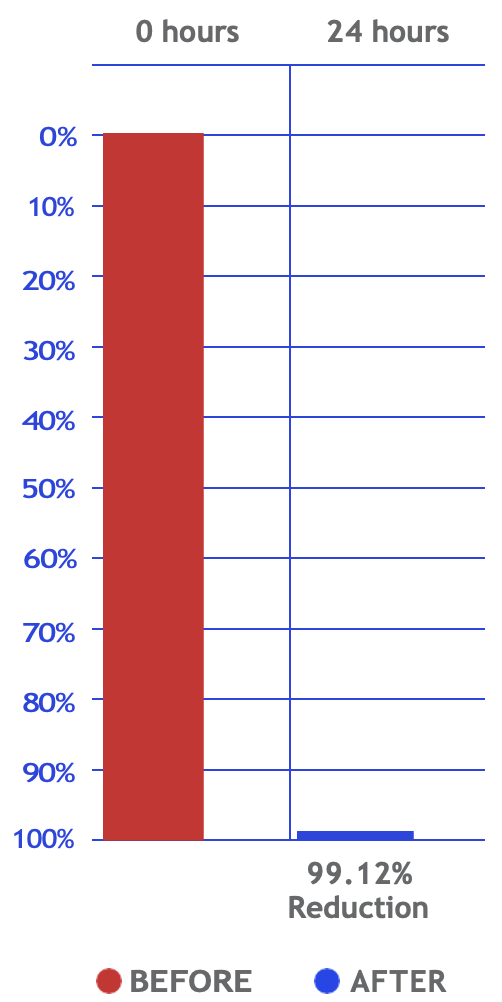
E coli Bacteria
Reduction E. coli
(Surface Test)
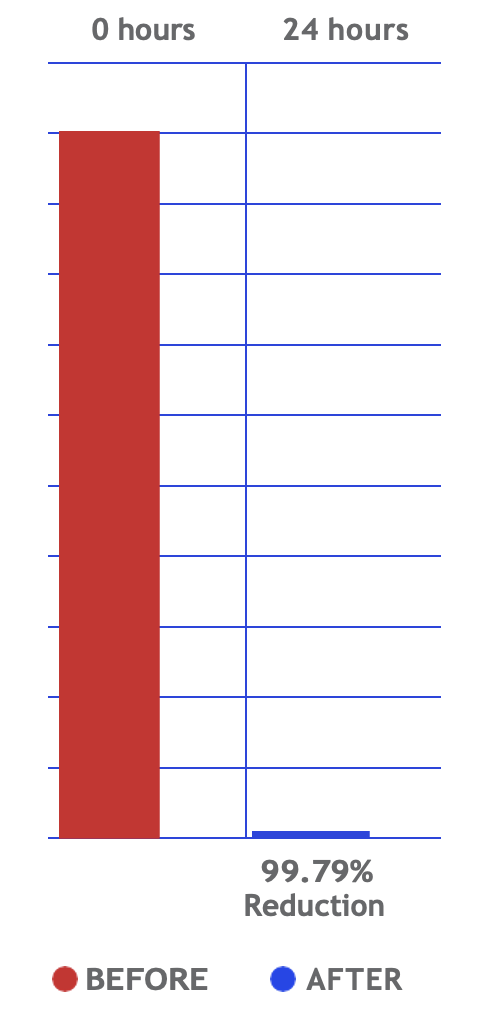
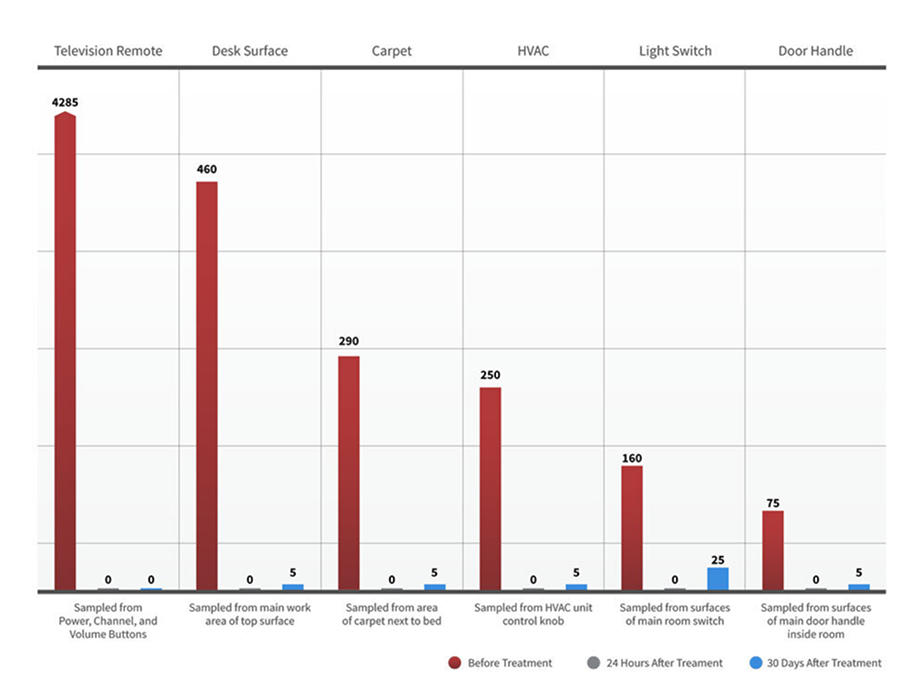
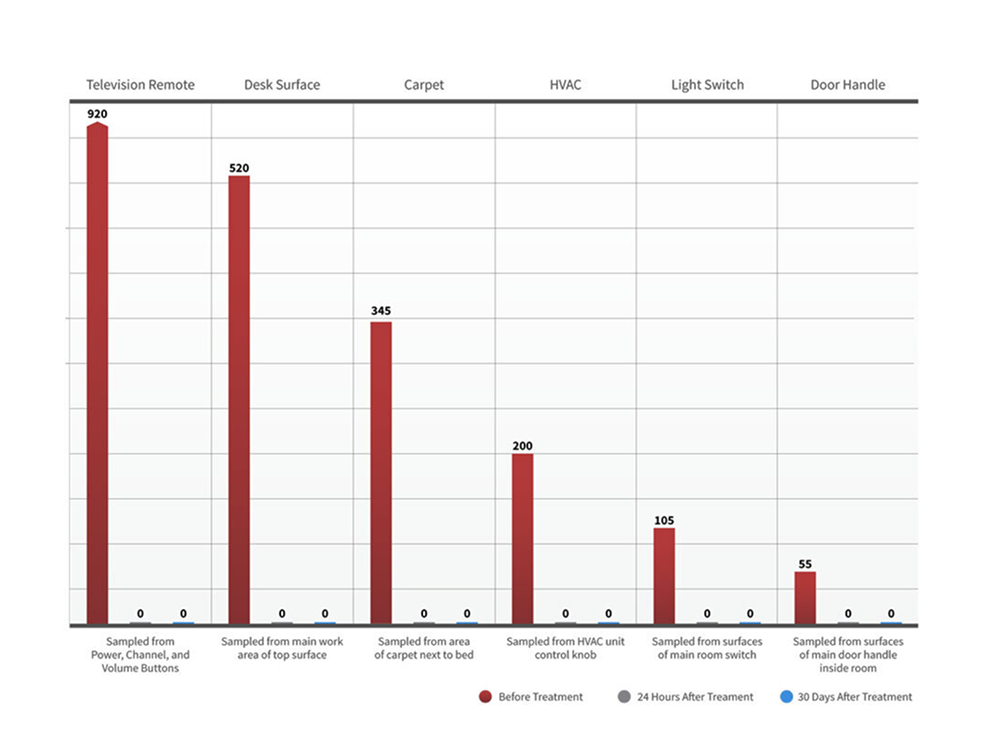
ActivePure® Medical Guardian has officially received FDA Class II Clearance as a medical device designed for continuous reduction of airborne and surface contaminants in professional health-care environments. Unlike passive systems that rely on filters or UV light, ActivePure® Technology works in real time to neutralize pathogens directly in the air and on surfaces, providing constant protection in occupied spaces.
Developed from NASA-inspired innovations, ActivePure® operates safely 24/7 without the use of ozone or harsh chemicals. The FDA-cleared unit has been scientifically validated to reduce a range of microorganisms, including staphylococcus epidermidis and erwinia herbicola bacteria; MS2 and Phi-X174 viruses; aspergillus niger fungal spores; and bacillus globigii bacterial spores under controlled clinical conditions. This positions ActivePure® Medical Guardian as the first and only FDA-cleared, real-time, active air purification system of its kind.
By eliminating contaminants at their source rather than merely capturing them, ActivePure® provides a higher level of protection for healthcare facilities, patient rooms, and other critical environments. This breakthrough clearance underscores the safety, effectiveness, and scientific credibility of ActivePure® Technology, making it a trusted solution for maintaining cleaner and healthier indoor spaces.
“I am pleased to be joining ActivePure Technologies, a company that provides a critical layer of protection against this virus, as well as other viruses, bacteria, and molds.”